Site pages
Current course
Participants
General
MODULE 1. Introduction to production of agricultur...
MODULE 2. Advance in material for tractor and agri...
MODULE 3. Advanced manufacturing techniques
MODULE 4. Heat treatment of steel
MODULE 5. Industrial lay out planning and quality ...
MODULE 6. Economics of process
MODULE 7. Techno economic feasibility of project r...
MODULE 8. Servo motors, drives and controllers
MODULE 9. CNC controllers for machine tools
MODULE 10. CNC programming
MODULE 11. Assembly and plant automation storage a...
LESSON 9. HEAT TREATMENT OF STEEL
1.1 Introduction
Heat treatment is a method used to alter the physical, and sometimes chemical, properties of a material. Heat treatment involves the use of heating or chilling, normally to extreme temperatures, to achieve a desired result such as hardening or softening of a material. Or Heat treatment is the controlled heating and cooling of metals to alter their physical and mechanical properties without changing the product shape.
Heat treatment is defined as an operation or combination of heating and cooling of a metal or alloy in the solid state for the purpose of obtaining desired change in properties. The heat treatment includes
-
Heating the metal to pre determined temperature
-
Holding the metal at that temp until the structure becomes uniform throughout the mass.
-
Cooling at pre determined rate to cause formation of desired change.
1.2 Objectives
-
To increase the hardness of metals
-
To relieve internal stress
-
To improve machinability
-
To improve mechanical properties like tensile strength, ductility and shock resistance
-
To change in grain size
-
To increase resistance to heat and corrosion
-
To modify electrical and magnetic properties
-
To change in chemical composition of metal surface
-
To remove gases
1.3 Types of heat treatment processes
1. Annealing
2. Normalizing
3. Hardening
4. Tempering
5. Case hardening
i. Carburizing
ii. Cyaniding
iii. Nitriding
6. Surface hardening
i. Induction hardening
ii. Flame hardening
7. Diffusion coating
1.3.1 Annealing
The process consists of slow heating the steel slightly above (30-50oC) the critical point (AC3) for hypo eutectoid (GS) and the same amount above PSK for the hyper eutectoid holding it at this temp for considerable period (3.4 min/mm length of piece); slow cooling in sand (the rate of cooling from 30oC to 200oC depends upon the composition of the steel. The grain structure has coarse Pearlite with ferrite or Cementite (depending on whether hypo or hyper eutectoid). The steel becomes soft and ductile.
Austenite - Pearlite and Ferrite (Hypo eutectoid)
- Pearlite (Eutectoid)
- Pearlite + Cementite (Hyper eutectoid)
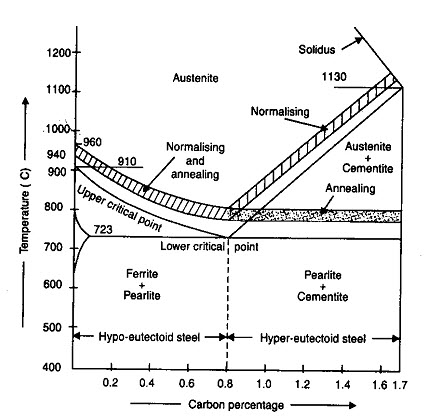
Fig. 4.1.1 Temperature ranges for various heat treatment process
The purpose of annealing may involve one or more of the following aims:
To soften the steel and to improve machinability.
To relieve internal stresses induced by some previous treatment (rolling, forging, uneven cooling).
To remove coarseness of grain.
To refine the grain size and structure to improve strength and ductility.
To alter electrical and magnetic properties.
1.3.2 Normalizing
It consists of heating the metal to a temperature just above the critical point (40-50oC) above AC3 line GS and ACm (GSE) within the normalizing range, holding it at this temp for period of 15 min and cooling in still air to room temperature. This process provides a homogeneous structure consisting of ferrite and pearlite for hypo eutectoid steels and pearlite and cementite for hyper eutectoid steels. This is done,
To produce a harder and stronger steel than full annealing
To refine the grain structure of steel to improve the machinability and tensile strength.
To modify and refine the grain structure
To obtain a relatively good ductility without reducing the hardness and strength
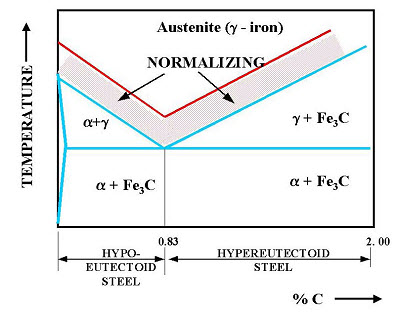
Fig. 4.1.2 Normalizing process
Hardness is a function of the carbon content of the steel. Hardening of a steel requires a change in structure from the body-centered cubic structure found at room temperature to the face-centered cubic structure found in the austenitic region. The steel is heated to autenitic region. When suddenly quenched, the martensite is formed. This is a very strong and brittle structure. When slowly quenched it would form austenite and pearlite which is a partly hard and partly soft structure. When the cooling rate is extremely slow then it would be mostly pearlite which is extremely soft.

Fig.4.1.3 Structure of steel when heated to critical stage
1.3.3 Hardening
It consists of heating the steel to a temperature above the critical point (30-50oC) above AC3 line; holding it at this temperature for a considerable period; quenching (sudden cooling) in water, oil/molten salt solution. The heating operation is required form the purpose of transforming the ferrite and pearlite for hypo eutectoid steels and pearlite and cementite for hyper eutectoid steels into austenite. A rapid cooling form the hardened temperature causes austenite to be transformed into martensite which is very hard and brittle.
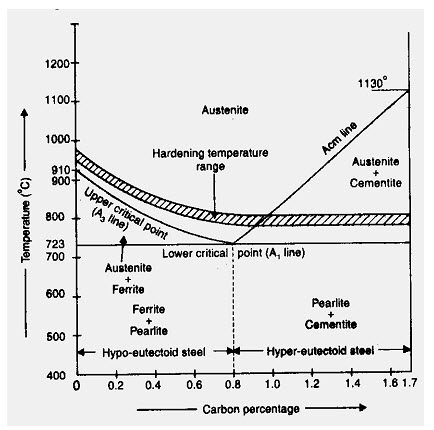
Fig.4.1.4. Hardening process
This is done to develop hardness to resist wear and to improve strength, elasticity, ductility and toughness and to enable it to cut other metals (to make it suitable for cutting tools).
1.3.4 Tempering
The steel hardened by rapid quenching is very hard and brittle. It also contains internal stresses, which are severe and unequally distributed to cause cracks or even rupture of hardened steel. Tempering is a process done subsequent to quench hardening. Quench-hardened parts are often too brittle. This brittleness is caused by a predominance of martensite. This brittleness is removed by tempering. Tempering results in a desired combination of hardness, ductility, toughness, strength, and structural stability. It consists of reheating the steel after hardening to a temperature below the critical point (PSK line); holding it for a considerable period and slow cooling (4-5 min/ mm). This is done to reduce the brittleness of the hardened steel and thus to increase ductility. to relieve the internal stresses and to make the steel tough to resist shock and fatigue.