Site pages
Current course
Participants
General
MODULE 1. Introduction to production of agricultur...
MODULE 2. Advance in material for tractor and agri...
MODULE 3. Advanced manufacturing techniques
MODULE 4. Heat treatment of steel
MODULE 5. Industrial lay out planning and quality ...
MODULE 6. Economics of process
MODULE 7. Techno economic feasibility of project r...
MODULE 8. Servo motors, drives and controllers
MODULE 9. CNC controllers for machine tools
MODULE 10. CNC programming
MODULE 11. Assembly and plant automation storage a...
LESSON 10. PACK CARBURIZING
2.1. Introduction
In engineering applications, it is desirable that steel being used should have hardened surface to resist wear. At the same time it should have soft and tough interior or core so that it is able to absorb any shock etc. Case hardening or surface hardening is the process of hardening the surface of a metal.
Case Hardening is a process of hardening ferrous alloys so that the surface layer or case is made substantially harder than the interior or core. The chemical composition of the surface layer is altered during the treatment by the addition of carbon, nitrogen, or both. The surface hardening processes are:
i. Carburising
ii. Cyaniding
iii. Nitriding
2.2. Carburizing
Carburizing is a process of adding carbon to the surface. It is the process of producing a hard surface on steel. It consists of heating the steel in the presence of carbonaceous material such as wood, bone, charcoal and energizers for increasing the concentrations of CO to improve rate of carburizing. Carbon enters the metal to form solid solution with iron and converts the outer surface into a high carbon case. Heating the composite steel to critical temperature cooling.
2.2.1. Pack Carburizing
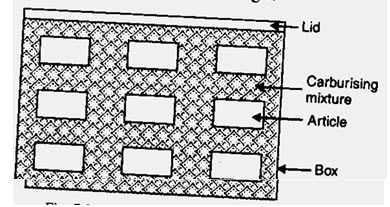
Fig. 4.2.1. Pack carburizing
Parts are packed in a high carbon medium such as carbon powder or cast iron shavings and heated in a furnace for 12 to 72 hours at 900 ºC. At this temperature CO gas is produced which is a strong reducing agent. The reduction reaction occurs on the surface of the steel releasing carbon, which is then diffused into the surface due to the high temperature. When enough carbon is absorbed inside the part (based on experience and theoretical calculations based on diffusion theory), the parts are removed and can be subject to the normal hardening methods.
2.2.2. Gas carburizing
Gas Carburizing is conceptually the same as pack carburizing, except that Carbon Monoxide (CO) gas is supplied to a heated furnace and the reduction reaction of deposition of carbon takes place on the surface of the part. This processes overcomes most of the problems of pack carburizing. The temperature diffusion is as good as it can be with a furnace.The only concern is to safely contain the CO gas. A variation of gas carburizing is when alcohol is dripped into the furnace and it volatilizes readily to provide the reducing reaction for the deposition of the carbon.
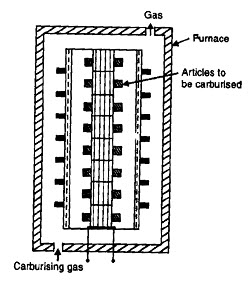
Fig. 4.2.2.Gas carburizing
2.2.3. Liquid Carburizing:
The steel parts are immersed in a molten carbon rich bath. In the past, such baths have cyanide (CN) as the main component. However, safety concerns have led to non-toxic baths that achieve the same result.
2.3. Cyaniding
Producing a hard surface on low carbon or medium carbon steel by immersing in a molten salt bath of cyanide at 800-900 oC and quenching in water or oil. Change in hardness is gradual, bright finish and distortion is avoided. This case hardening process heats ferrous materials above the transformation temperature in a molten salt bath containing cyanide. The absorption of both carbon and nitrogen at the surface also produces a gradient in from the surface. Subsequent cooling is specified to produce the required hard, wear-resistant properties.
2.4. Nitriding
Nitriding is a surface-hardening heat treatment that introduces nitrogen into the surface of steel. The process involves heating the steel in an atmosphere of ammonia gas at 560oC to 650oC without further heat treatment. Results in extreme hardness. Used in automotive, and diesel engine wearing parts. Principal reasons for nitriding are:
To obtain high surface hardness
To increase wear resistance
To improve fatigue life
To improve corrosion resistance (except for stainless steels)
To obtain a surface that is resistant to the softening effect of heat at temperatures up to the nitriding temperature.
In this process, nitrogen is diffused into the surface of the steel being treated. The reaction of nitrogen with the steel causes the formation of very hard iron and alloy nitrogen compounds. The resulting nitride case is harder than tool steels or carburized steels. The advantage of this process is that hardness is achieved without the oil, water or air quench. As an added advantage, hardening is accomplished in a nitrogen atmosphere that prevents scaling and discoloration.
Nitriding temperature is below the lower critical temperature of the steel and it is set between 496 oC and 566oC. The nitrogen source is usually Ammonia (NH3). At the nitriding temperature the ammonia dissociates into Nitrogen and Hydrogen. The nitrogen diffuses into the steel and hydrogen is exhausted. A typical nitriding setup is illustrated in Fig.
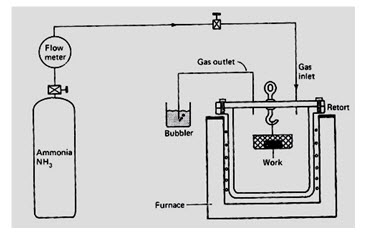
Fig. 4.2.3.Nitriding setup
2.5. Surface hardening
Differs case hardening in that analysis of surface steel is not changed, hardening being by extremely rapid heating and quenching of wearing surfaces which has no effect on the interior core.
Induction hardening
Rapid heating on medium carbon steel by induced current high frequency and rapid cooling by sprays of water. Used on bearing areas of crank shaft, cam shaft, axial shaft etc., In this process an electric current flow is induced in the workpiece to produce a heating action.
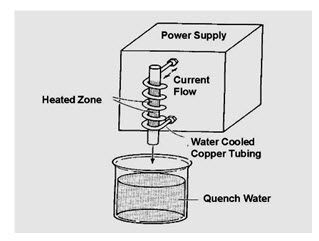
Fig. 4.2.4. Induction hardening
Every electrical conductor carrying a current has a magnetic field surrounding the conductor. Since the core wire is a dead-end circuit, the induced current cannot flow anyplace, so the net effect is heating of the wire. The induced current in the core conductor alternates at frequencies from 60 cycles per second (60 Hz) to millions of Hertz. The resistance to current flow causes very rapid heating of the core material. Heating occurs from the outside inward. Induction hardening process includes water quench after the heating process. The big advantage of this system is its speed and ability to confine heating on small parts. The major disadvantage is the cost.
Flame hardening
Surface hardening process in which heat is applied by a high temperature flame followed by quenching jets of water. It is usually applied to medium to large size components such as large gears, sprockets, slide ways of machine tools, bearing surfaces of shafts and axles, etc. Steels most suited have carbon content within the range 0.40-0.55%. Heating the steel with flame of oxyacetylene torch and quenching.
Flame hardening is the process of selective hardening with a combustible gas flame as the source of heat for austenitizing. (The material should have at least 0.40 % carbon content to allow hardening.) Water quenching is applied as soon as the transformation temperature is reached. The heating media can be oxygen acetylene, propane, or any other combination of fuel gases that will allow reasonable heating rates. This procedure is applied to the gear teeth, shear blades, cams, ways on the lathes, etc. Flame hardening temperatures are around 816 oC.
2.6. Diffusion coating
A coating process used to change the surface composition of a metallic material with (1) another metal or alloy employing heat or (2) exposure to a gaseous or liquid metal to effect diffusion into the basis metal. Impregnating the surface of steel with aluminium. chromium, silicon, beryllium and other elements. Impart high heat, corrosion and wear resistance. Heating and holding the steel parts in direct contact with one of the above elements. Diffusion coatings provide superior oxidation, corrosion and erosion resistance of the base metal up to 11500 C without spallation. Diffusion coating is a highly reliable, substate enhancing process for critical components.
2.7. Precipitation hardening or Age hardening
A process of aging that increases hardness and strength and usually decreases ductility. Hardening in metals caused by the precipitation of a constituent from a supersaturated solid solution. Increasing the hardness of an alloy by a relatively low temperature heat treatment that causes precipitation of components or phases of the alloy from the supersaturated solid solution. Also known as precipitation hardening.
Precipitation hardening, also called age hardening, is a heat treatment technique used to strengthen malleable materials, including most structural alloys of aluminium, magnesium and titanium, and some stainless steels. Spontaneous increase in hardness at room temperature with lapse in time. Precipitation is the decomposition of a solid solution into two phases of different composition viz., precipitate and solid solution. Precipitation hardening is caused by precipitation of a constituent from a super saturated solid solution by heating to some elevated temperature. In order that metals may serve useful purposes they must be shaped into required forms.