Site pages
Current course
Participants
General
Module 1. Introduction to by-products and waste ge...
Module 2. Waste management concepts
Module 3. Direct combustion of solid waste
Module 4. Thermo-chemical conversion of solid waste
Module 5. Bio-chemical conversion of solid waste
Module 6. Solid waste management
Module 7. Effluent treatment and disposal
Module 8. Presence of typical chemicals
19 April - 25 April
26 April - 2 May
Lesson 10.
Parameters of effluent
Temperature
It affects the activity of bacteria present in the waste water along with solubility of various gases in it. Further, based upon solubility, it may affect viscosity of sewage thus having an impact on sedimentation part of waste water. On an average of 20oC temperature is observed for waste water suitable from microbial activity point of view. However, when temperature is more, the D.O. content of waste water is reduced considerably.
pH
pH value of a solution may indicates negative log of hydrogen ion concentration present in waste water.
Mathematically, it can be expressed as:
pH = - Log10H+
H+ = 10-pH
Thus it indicates alkalinity/acidity of sewage
pH > 7 → Basic solution
pH = 7 → Neutral solution
pH < 7 → Acidic solution
The determination of ph of waste water helps us to choose suitable mode of treatment as suitability of treatment or to be more precise, efficiency of treatment depends upon ph value. So, it is very important parameter for waste water treatment. The ph of fresh sewage is generally falls because of production of acids by bacterial actions. It has been observed that with the passage of time, once sewage gets stabilized, ph starts increasing.
Presence of Fats, Oils and Grease
Fats, oils and grease may find its origin in waste water by discharges of animal/vegetable matter, or from the kitchens, hotels or restaurants, garages, milk plants, industries etc. This type of materials form scum on the top of sedimentation tanks and clog the pores of various filters. So, it need to be properly detected and checked at source before the waste water is being fed into treatment plant for sedimentation. To measure the quantity of fats and oils, a sample of sewage is evaporated. The residual solids left are then mixed with ether. The solution is then poured off and again evaporated. The residual left now behind will be fats and greases and thus can be easily removed by means of grit chambers or detritions tanks.
Oxygen demands
The availability of oxygen in water or waste water is very important. If the waste water has to be directly discharged into a water body/river, it should be able to provide dissolved oxygen of a minimum of 4 mg/l. The dissolved oxygen test performed on sewage prior to its treatment helps us to decide mode as well as degree of treatment. Moreover, it will let us know about the state of sewage means whether it is fresh or stale. Its only fresh sewage that contains a little of dissolved oxygen. It can be determined by Winkler’s method. Two types of oxygen demands for waste water has to be determined that is BOD and COD.
BOD
It is Bio-Chemical Oxygen Demand. The waste water contains two types of matter. One that can be easily oxidized by the bacteria and thus called biologically active or degradable matter. The other part of material present in waste water cannot be degraded biologically and thus known as biologically inactive. BOD of sewage gives us the amount of biologically active organic matter present in sewage and is one of the most important tests of waste water as treatment processes/methodology that need to be adopted to it is based upon this value.
If good amount of oxygen is present in water then the aerobic biological decomposition of waste water will take place until the oxidation process is completed. The amount of oxygen consumed in this process is nothing but BOD. The bacteria present in sewage will keep on absorbing oxygen and the process of decomposition will keep on going for a number of months. It is practically not feasible to determine ultimate oxygen demand. So, BOD of water or waste water during 5 days at 20oC is generally taken as standard demand. It is about 68 % of the total demand. The standard BOD 5 day demand is designated as BOD5 and is found in laboratory by diluting a known volume of a sample of waste water with a known volume of aerated pure water (dilution water) and then finding D.O. of this diluted sample. The diluted sample is then incubated for 5 days at 20oC. The dissolved oxygen of the diluted sample after 5 days is again calculated. This difference will indicate the O2 consumed by sewage sample in 5 days. BOD5 is thus calculated as
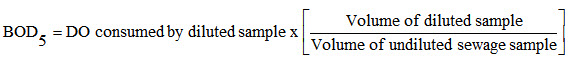
The factor in the right hand side in the above equation is nothing but dilution factor.
The rate at which BOD is deoxygenated depends upon amount and nature of organic material present in the sample and also on temperature of waste water.
Mathematically,
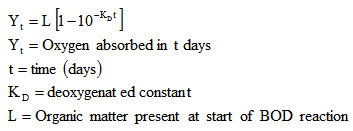
COD
The amount of oxygen required to decompose both biologically active matter and biologically inactive matter is known as chemical oxygen demand or COD. It can be calculated in laboratory by performing tests in a number of ways. The most commonly used is by using a strong oxidant like potassium dichromate K2Cr2O7 or KMnO4 to stabilize the organic matter to determine the molecular oxygen from the oxidant solution in oxidizing the organic matter present in the given waste water.
Metal Content
Waste water treatment systems for metals are pretty well defined for precipitation systems. The incoming solution is ph adjusted to the optimum range for precipitating metal as a hydroxide. In difficult situation a sulfide is added to increase recovery. The treated water is run through a clarifier to settle solids.
The standard heavy metal waste water treatment system adopts hydroxide precipitation for heavy metal removal. It may be aided with or without addition of sulfides. The sulfides results in a lower solubility than hydroxide precipitation alone.
Phosphorous
Municipal waste waters may contain 5-20 mg/l of phosphorous. Out of this 20-25% is organic and rest is inorganic. The usual forms of phosphorous found in aqueous solutions include:
- Orthophosphates: Available for biological metabolism without further break down.
- Polyphosphates: Molecules with 2 or more phosphate atoms, O2 and some cases H2 atoms combine to form complex substances.
Polyphosphates may get hydrolyzed and transform into orthophosphates; but this process is very slow. Phosphates can be removed by means of phosphate precipitation by addition of coagulant. The multivalent ions commonly used are Ca, Al and Fe. Normally secondary treatment systems can remove only 1-2 mg/l of phosphorous. Thus a large excess of phosphorous is discharged in final residues causing eutrophication in surface waters. New legislation require a maximum concentration of P discharges into sensitive waters of 2 mg/l.
Sulphur
It may be present in the form of sulphides, sulphates and H2S in waste water. The determination of sulphur in these forms is rarely done however their presence may indicate aerobic and /or anaerobic decomposition. Sulphides and sulphates may be formed due to decomposition of various sulphur containing substances present in sewage; resulting in production of H2S (↑) and thus a very foul smell and odour. It may also cause corrosion of sewer pipes through it is carried away. If H2S quantity in waste water is less than 1 ppm, it is hardly felt. Moreover sulphides and H2S indicate initial stages of decomposition. Once the decomposition is over, only end products in form of sulphates are available which are comparatively stable and unobjectionable.