Site pages
Current course
Participants
General
Module 1. Design and operational parameters
Module 2. Performance evaluation and maintenance a...
Module 3. Performance evaluation and maintenance a...
Module 4. Performance evaluation and maintenance a...
Module 5. Performance evaluation and maintenance a...
Module 6. Performance evaluation and maintenance a...
Module 7. Biodiesel utilization in CI engines
Lesson 3. Characteristics determination of biomass
Agricultural biomass which includes crop residues and weeds are a source of renewable energy with good scope or exploitation through different conversion routes. Section of suitable energy conversion route is very essential for the efficient conversion of biomass.
In order to effectively utilize the biomass, their characteristics are to be assessed. To asses the suitability of biomass for conversion through any of the processes, characterisation of physical (moisture content, density, etc.,) chemical (proximate and ultimate composition, trace elements content etc.) and thermal properties (calorific value, tar content, charcoal content etc.) of biomass is essential. Classification of commonly available species for their suitability based on their characteristics is important for their proper selection in different applications such as thermo chemical, biochemical and other conversion processes.
A. Physical Characteristics
-
Density
-
Moisture content
B. Chemical Characteristics
(i) Proximate composition
Volatile content
Ash content
Fixed carbon
(ii)Ultimate composition
Elemental carbon
Elemental hydrogen
Elemental nitrogen
Elemental oxygen
Elemental sulphur
(iii)Trace elements
C. Thermal Characteristics
-
Calorific value
-
Condensable tar yield
-
Charcoal yield
-
Temperature of maximum devolatilisation
The methodologies for the characterisation of the biomass materials are given below.
Physical Characteristics
Physical characteristics are useful to estimate the portion of biomass available for energy conversion.
Density
The specimen whose density is to be measured has to be made into a regular geometrical shape. An electronic balance (Fx/Fy series) having provision to weigh using the built-in ‘under hook’ may be used. The under hook is to be fitted through the lower end of the riser beam directly below the pan support peg of the balance. A light-weight strand of thin rod will be hung through the hook. After placing the balance on a firm metal stand designed for under hook weighing, the weight of the regularly shaped specimen in air will be taken. Then the sample has to be fixed in the lower end of the hook and allowed to immerse in glycerol and the weight is to be taken. The density of the specimen will be then worked out from the formula:
![]()
Moisture Content
For the moisture content determination, known weight of sample is to be dried in an open petry dish in an electrical oven at 103 +/- 50 C for 1 hour and weighed. It is assumed that this oven dried weight indicated a condition of zero moisture content. The difference in weight, before and after drying indicates the moisture content of each sample. As the moisture content of biomass differs from each other, oven dry sample are taken for analysis of all the other properties. The moisture content can be determined as follows:
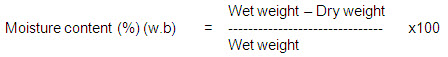
Chemical Characteristics
The chemical constituents of the biomass materials can be determined (proximate, ultimate composition and trace elements) as per the procedures given below.
Proximate Composition
Proximate analysis gives information of biomass constituents such as volatiles, fixed carbon and ash content of water free samples.
Volatile Matter
Volatile matter can be determined by keeping the dried sample in a closed crucible at 6000 C for six minutes and then at 750o for another six minutes. The difference in the weights due to the loss of volatiles will be considered as the total volatile matter present in the sample.
Ash Content
The samples may be taken in a silica crucible and heated gradually in a muffle furnace to 750o C for two hours or more till recording constant weight. The weight of the residue represents the ash content.
Fixed Carbon
The fixed carbon may be found out by subtracting the sum of percentages of ash content and volatile matter from 100.
Element Analysis
This analysis has to be carried out to determine the content of carbon, hydrogen, nitrogen, potassium, sodium, calcium, magnesium and phosphorus in the sample. These amounts are expressed as percentage of the weight of the water free sample. The methods of determination for each element are described below.
Instrumental Method
The ultimate analysis of samples may be carried out using Elemental Analyser. The element C, H, N and S are determined using the instrument and the element O may be found by difference.
Analytical Method
The analytical methods for the determination of Carbon, Nitrogen, Hydrogen and other elements are described below.
Carbon
For the determination of carbon, Tyurin method can be adopted. In this method a known amount of sample (about 10 g) is placed in a 100ml conical flask. Ten ml. of 0.4 N potassium dichromate solution is added to the flask. A 4cm diameter funnel is kept on the flask, which is heated to allow gentle boiling of these contents for 5 minutes. After boiling, the flask is allowed to cool. The funnel and the flask are then rinsed with 10 ml of distilled water. The orange yellow colour pf the liquid in the flask after rinsing is an indication of complete oxidation of carbon. If the colour is found to be a greenish brown, it is an indication of incomplete oxidation and the reaction is repeated using larger amounts of potassium dichromate. The unused quantity of potassium dichromate in the content of the flask after the reaction is determined by titrating the contents against 0.1 N
Mohr’s salt using phenyl antheranilic acid as indicator. For blank same amount of potassium dichromate is heated under similar conditions and titrated against 0.1 N Mohr’s salt. The percentage of carbon in the sample is calculated from the following equation:
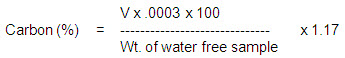
Where V is the difference in volume of Mohr’s salt used for blank and test runs.
Nitrogen
Modified Kjeldahl method is used to determine the nitrogen content. One gram air dried and ground sample (-50+100 mesh ASTM) is mixed with 5 g of digestion mixture (K2SO4 : CuSO4 : 1:10). The mixture is put in a 300 ml Kjeldahl digestion flask and 25 ml of concentrated H2SO4 is added to the contents of the flask. The flask is heated for 3-4 hours till the digestion is completed. Appearance of light green colour in a transparent solution is an indication of complete digestion. The digested mixture is diluted with distilled water to make the volume up to 100ml. Two ml of diluted mixture is taken in Kejldahl distillation apparatus and mixed with 5 ml of 40% NaOH. Vapours are absorbed in 4% boric acid. The solution thus obtained is titrated against 0.01 N H2SO4 using mixed indicator (bromocresol green 0.5% and methyl red 0.1% in ethyl alcohol).
Nitrogen content is calculated by the following equation.
Nitrogen % = V x 0.7 / weight of sample
Where V is the volume of .01 N H2SO4 used.
The value has to be corrected to be expressed as per cent of weight of water free sample.
Hydrogen
It is determined by combustion method. One gram of air dried and ground sample is oxidized by heating it in oxygen atmosphere in the presence of cupric oxide. The resultant water vapours are absorbed in dehydrated calcium chloride. The amount of hydrogen may be calculated from the weight of the water vapours. Appropriate correction factor is used to take into account the initial moisture content of the sample and the hydrogen content of the sample can be expressed as per cent of the weight of the water free sample.
Determination of Potassium, Sodium, Calcium, Magnesium and Phosphorus
For the determination of potassium, sodium, calcium, magnesium and phosphorus content, 0.5 g sample is digested with 15ml of acid mixture (HNO3 : HCLO4, 3:1) in a 100 ml conical flask. The digested mixture is analyzed with flame photometer using appropriate filters for the determination of potassium and sodium. The values are corrected to be expressed as per cent of the water free residue sample. The digested mixture, after adequate dilution is analysed on atomic absorption spectrometer for the determination calcium and magnesium. The values are corrected to be expressed per cent of moisture free sample. For the determination of phosphorus the digested mixture is analysed on spectrophotometer.
For the determination of silica, 1 g of air dried and ground sample is treated with 40 ml of hydrochloric acid of 50% concentration in a china dish which is heated for 30 minutes on a water bath. One ml of concentrated HNO3 is then added to the contents 0 of the dish, which is again heated on a burner/hot plate till the liquid evaporated completely leaving only dry residue. The dry residue is heated on a water bath for 30 minutes after which 10 ml HCL of 50% concentration is added to it. The mixture is stirred and 50 ml of distilled water mixed with it. The mixture is filtered through what man No.44 filter paper. The residue along with filter paper is kept in weighted silica crucible and heated in a furnace for 2 hours at 700o C. The crucible is cooled in a desiccators and weighed to determine the amount of silica. The silica is expressed as per cent of the weight of water free sample.
Thermal Properties
The procedures followed for the determination of the other properties like, calorific value, condensable tar yield temperature of maximum devolatilisation and charcoal yield are given below.
Calorific Value
Calorific value (HHV) of most of the biomass reported is determined in bomb calorimeter. Calorific value can also be found out using either by Jenkins and Ebeling formula or by Dulong’s formula:
CV= (0.293 C + 5.21) MJ kg-1 jenkins and Ebeling formula
Where C is the per cent composition of element carbon.
CV= (8080 C + 34500 (H-(0/8)) +2240 S) kcal kg-1 Dulong’s formula.
Where C, H, O and S are fractional composition of element carbon, hydrogen and oxygen and sulphur respectively.
Condensable tar yield and temperature of maximum devolatilisation
This is a simple approximate procedure for estimating condensable tar yield and temperature of maximum devolatilisation. The experimental set up which has a covered cup with a stem, is used. A known weight of sample is taken in the cup. This set up is kept in a programmable furnace. The tar released at varying heat rate is condensed by condensers. The tar released is collected at temperature intervals of 0-200o C, 200-400o C, 400o -600o C, 600o -800o C and 800o -1000o C separately. Then the final weight of the sample and tar collected can be observed.
The peak temperature corresponding to maximum tar yield is taken from a plot of condensable tar yield against temperature to be the approximate maximum devolatilisation temperature.
Charcoal Yield
The sample of known weight (say 50 g) is taken in the closed container. It is kept in a muffle furnace for specified time at 200oC - 350oC. Then the container is cooled and the charcoal is weighed. The percentage of charcoal yield is calculated.
Based on the characteristics of the biomass, suitable conversion technologies can be adopted for efficient energy production from the available sources.