Site pages
Current course
Participants
General
Module 1. Design and operational parameters
Module 2. Performance evaluation and maintenance a...
Module 3. Performance evaluation and maintenance a...
Module 4. Performance evaluation and maintenance a...
Module 5. Performance evaluation and maintenance a...
Module 6. Performance evaluation and maintenance a...
Module 7. Biodiesel utilization in CI engines
Lesson 4. Stochiometric air requirement calculation
Every material requires oxygen for combustion. If a material has to be completely combusted, it requires specific amount of oxygen. The specific amount will be calculated based on the elemental composition of the material and is named as stochiometric oxygen requirement. As air is the cheapest source of oxygen, normally air will be supplied for combustion of materials unless it is warranted. Thus stochiometric air requirement is an important factor in achieving complete combustion of a material.
The procedure for calculating the stociometric air requirement of a material whose elemental composition such as carbon, hydrogen, nitrogen, oxygen and suphur are known is given below.
Carbon

In burning I kg of carbon needs 8/3 kg of oxygen to produce 11/3 kg of CO2
If the oxygen supply is insufficient then the equation may be
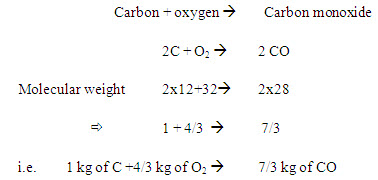
In burning I kg of carbon needs 4/3 kg of oxygen to produce 7/3 kg of CO
Carbon monoxide
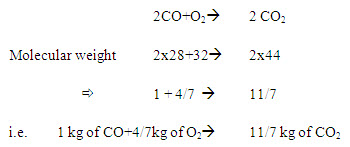
In burning I kg of carbon monoxide needs 4/7 kg of oxygen to produce 11/7 kg of CO2
Hydrogen

In burning I kg of hydrogen needs 8 kg of oxygen to produce 9 kg of H2O
Sulphur

In burning I kg of sulphur needs 1 kg of oxygen to produce 2 kg of SO2
Methane
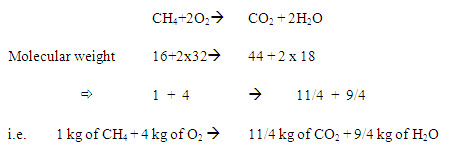
In burning I kg of methane needs 4 kg of oxygen to produce 11/4 kg of CO2 and 9/4 kg of water vapour
For complete combustion minimum amount of oxygen requirement by elements
1 kg of carbon 8/3 kg
1 kg of hydrogen 8 kg
1 kg of sulphur 1 kg
Total oxygen requirement = 8/3 C + 8 H2 + S
Oxygen available in fuel = O2
Net oxygen requirement is = 8/3 C + 8 H2 + S - O2
Directly supply of pure oxygen of combustion process is not economical and also not necessary. Hence naturally, abundantly and freely available oxygen source, air can be supplied for combustion of raw materials. While considering the amount of oxygen in air is 23% by weight and 21% by volume. Considering weight fraction the amount of air required for combustion of the fuel material is given by
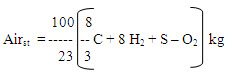
If the theoretical sir supply is supplied, some amount of fuel may be unburnt. Air supplied may not come into intimate contact with the fuel particles; there by excess sir supply is required for complete combustion. This excess air is depends on the quantity of material, rate of combustion, firing system etc. Generally 25 to 50 per cent excess air will be supplied. The supply of excess air produces cooling effect. But this can be avoided by preheating the air before its supply for combustion.
Total air supplied (Airtot) = Airst + Airex
Products of combustion
Similarly, the quantity of products of combustion may be calculated through the above methodology. The products such as carbon dioxide from carbon, water vapour from hydrogen, sulphur dioxide form sulphur, excess oxygen and uncombusted remaining nitrogen can be calculated using the formulae given in the table.
|
Products of combustion |
Amounts of products of combustion |
Percentage by mass (m/∑mx100), % |
|
|
Formula |
Mass,m, kg |
||
|
CO2 |
11/3 x C |
m1 |
m1/∑m x100 |
|
H2O |
9 H2 |
m2 |
m2/∑m x100 |
|
SO2 |
2 S |
m3 |
m3/∑m x100 |
|
O2in excess air |
0.23 X mex |
m4 |
m4/∑m x100 |
|
N2 in total air |
0.77 x m0.77 |
m5 |
m5/∑m x100 |
|
|
|
∑ m |
100% |
The excess oxygen gives an idea about energy efficiency of the combustion process. More amount of oxygen in the combustion product will indicate additional supply of oxygen than requirement and inefficient combustion. In this case, not only higher energy is added for the supply of oxygen but also, the excess oxygen will carry some quantity of heat of combustion to the atmosphere and will lead to reduced heat supply to the system.