Site pages
Current course
Participants
General
Module 1. Basic Concepts, Conductive Heat Transfer...
Module 2. Convection
Module 3. Radiation
Module 4. Heat Exchangers
Module 5. Mass Transfer
Lesson 18. Radiation
As discussed earlier in Lesson 1, heat is transferred between two bodies even though they are not in direct physical contact and vacuum exists between these bodies. Such a heat transfer is termed as radiation heat transfer that involves emission of energy by heat exchanging bodies due to changes in electronic configurations of their molecules or atoms. Energy is emitted in the form of electromagnetic radiation. The electromagnetic radiation that can be detected as heat is termed as thermal radiation. Thermal radiation emitted by a body or substance is directly proportional to its temperature. Thermal Radiation is one of the modes of heat transfer and it differs from conduction and convection modes of heat transfer in following aspects:
i) Conductive and convective heat transfer depends upon temperature difference (raised to power one) between heat exchanging bodies whereas in radiation mode, heat transfer is directly proportional to the difference of the fourth power of absolute temperatures of individual bodies.
ii) Presence of an intervening medium between two bodies, exchanging heat by radiation mode, is not necessary. Rather, heat transfer by radiation mode takes place most efficiently in vacuum. In case of heat transfer by conduction and convection modes, presence of intervening medium is a must for exchange of heat between two bodies.
iii) Heat transfer by conduction and convection takes place from high temperature body to low temperature body. In radiation mode of heat transfer, thermal energy is not only emitted by high temperature body towards low temperature body but also from low temperature body towards high temperature body. However, net transfer of energy is always from high to low temperature body.
Basic Theory of Radiation Heat Transfer:
Transfer of heat by from the Sun to the earth by thermal radiations is the prime source of energy and without which survival of mankind can not be imagined. Two theories have been postulated to explain the phenomenon of heat transfer by radiation.
i) Electromagnetic Wave Theory
ii) Quantum Theory
i) Electromagnetic Wave Theory
The electromagnetic wave theory of radiation was first postulated by James Clerk Maxwell in 1864. According to this theory, heat transfer by radiation mode takes place as thermal energy is emitted by a body in the form of electromagnetic waves due to change in electronic configuration of its molecules or atoms. The emitted electromagnetic waves travel through space that is assumed to be filled with an hypothetical medium called ether and are absorbed by another body.he absorbing body reconverts these electromagnetic waves in to thermal energy. Re-conversion of energy carried by electromagnetic waves into thermal energy depends upon the material and surface characteristics of the absorbing body. Internal energy of emitting body decreases as reflected by decrease in its temperature with a corresponding increase in temperature of the absorbing body resulting in increase in its internal energy.
ii) Quantum Theory
Max Planck postulated quantum theory of radiation in 1900. According to this theory, molecules and atoms of a hot body are in excited state due to high energy levels. These high energy level molecules and atoms tend to return to low energy levels and during this process, the body emits energy in the form of electromagnetic radiations. The energy is not emitted continuously but radiated in form of successive and separated quantities which are termed as quanta or photons. The size of each quantum is different and energy of quantum is expressed as
E = h ν (1)
Where h is Planck’s constant and is equal to 6.62 X 10-34 J-sec
ν is frequency of vibrations and is directly proportional to temperature of body
Spectrum of Electromagnetic Radiation:
In nature, energy is transferred from one place to another place by electromagnetic radiation. Electromagnetic radiation travel through space and has electrical and magnetic effects. Electromagnetic radiation of different types exists and one type differs from the other on account of its frequency and wavelength. The spectrum measures range of frequencies of all electromagnetic radiation and it includes
Radio
Microwaves
Infrared
Visible
Ultra-violet
X-rays
Gamma rays
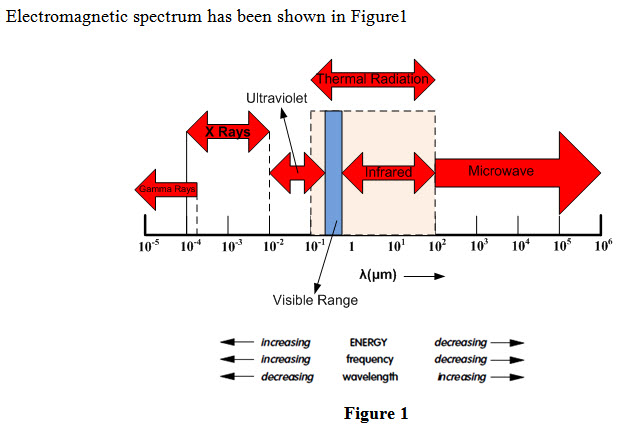
Every substance having temperature above absolute zero temperature emits electromagnetic radiation which is termed as thermal radiation. The rate of thermal radiation emission increases with increase in temperature of a substance. Range of wavelength of thermal radiation is between 0.1 to 100 μm and includes entire visible and infrared (IR) radiation as well as a portion of ultraviolet (UV) radiation. Following properties of thermal radiations are important from heat transfer point of view:
i) Thermal radiation travel through space in straight lines and do not heat up the space unless they are obstructed in their path.
ii) Nature and behavior of thermal radiation is same as that of visible light and they differ in wavelength only.
iii) Similar to visible light, thermal radiation obeys inverse square law and are also reflected as well as refracted.
Reflection, Absorption and Transmission of Radiation:
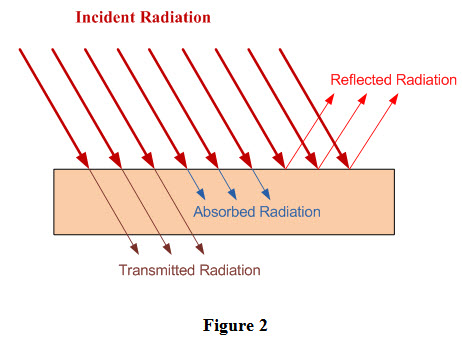
Thermal radiation is emitted by all substances and magnitude of emitted thermal radiation depends upon temperature of the emitting substance. Thermal radiation received by a body is partly absorbed, partly reflected and partly transmitted through the body. It has been shown in Figure 2 that Q amount of thermal radiation is incident upon a body, out of which Qt amount is transmitted through the body, Qr is reflected by the body and Qa is absorbed by the body. Therefore,
Qt+ Qr+ Qa = Q (2)
Dividing both sides of equation (2) by Q, we get
\[\frac{{{Q_t}}}{Q} + \frac{{{Q_r}}}{Q} + \frac{{{Q_a}}}{Q} = \frac{Q}{Q}\] (3)
\[\tau+ r + \alpha= 1\]
Where \[\tau \] is transmissivity of the body and is equal to \[\frac{{{Q_t}}}{Q}\]
r is reflectivity of the body and is equal to \[\frac{{{Q_r}}}{Q}\]
\[\alpha \] is absorptivity of the body and is equal to \[\frac{{{Q_a}}}{Q}\]
Absorptivity, transmissivity and reflectivity are dimensionless properties of the receiving body and values of these vary from 0 to 1.
Transmissivity of most of the solids and liquids is zero; however, there are few exceptions such as glass which allows transmittance of thermal radiation through it.
Therefore, for solids and liquids,
α + r = l
Depending upon values of absorptivity, reflectivity and transmissivity, bodies are classified as
|
Type |
Absorptivity, α |
Transmissivity, τ |
Reflectivity, r |
|
Black Body |
1 |
0 |
0 |
|
White Body |
0 |
0 |
1 |
|
Transparent Body |
0 |
1 |
0 |
|
Opaque Body* |
Less than 1 |
0 |
Less than 1 |
|
For Most of Gases** |
Less than 1 |
Less than 1 |
0 |
* α + r = l
** α + τ = l
Reflection of Radiation: Radiation reflection can be categorized as
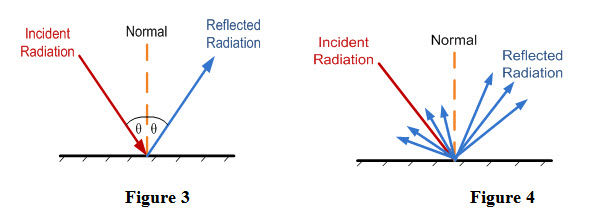
- Specular Reflection: If angle of reflection is equal to angle of reflection, then it is called specular reflection as shown in Figure 3. Specular reflection occurs if surface of receiving body is highly polished.
- Diffused Reflection: If incident thermal radiation is reflected in all directions as shown in Figure 4, then it is called diffused reflection. Diffused reflection occurs if surface of receiving body is rough.